No more Garbage in , Garbage Out.
The medical device industry is increasingly embracing computational modeling and simulation (CM&S) as a valuable tool in the development process. CM&S offers advantages, including reduced reliance on animal testing, cost savings potentially, and shorter development timelines. The most treasure is the prediction capability from those models. However, a critical challenge lies in establishing the credibility of these models and their results, especially when seeking approval from regulatory bodies such as the FDA.
This is where the ASME V&V 40 standard comes into play. Published by the American Society of Mechanical Engineers(ASME), V&V40 provides a structured framework for demonstrating the credibility of CM&S models used in medical device development. This blog post is going to provide a deep understanding of V&V40, exploring its key components and concept of risk, implementation challenges, and who are encouraged to engage this standard.
The Scope and Purpose of V&V40
The primary objective of V&V40 is to establish confidence in the reliability of computational models used in medical device development. This is achieved by providing a framework for assessing the model’s verification, validation, and applicability. This comprehensive assessment helps demonstrate the model’s trustworthiness and suitability for decision-making under its context of use. It’s important to note that V&V40’s focus is on physics-based modeling, meaning it only supports those models which was established by laws of physics to simulate real-world phenomena. This standard excludes other modeling approaches, such as statistical modeling, machine learning, and AI models, which may require different validation methods. People asked about the availablity of V&V40 for pharmaceutical sciences, like PK/PD modeling. So far, this sector was not stated in V&V40. In brief, if your modeling or simulation is physics-based and for medical devices, then this article is for you.
A Framework for Building Trust
The V&V40 standard emphasizes a risk-based approach to evaluating model credibility, moving away from simply labeling a model as “validated.” The standard outlines key elements that are crucial for establishing a model’s credibility. Before that, you will need to have fully understanding the context of use of your simulation.
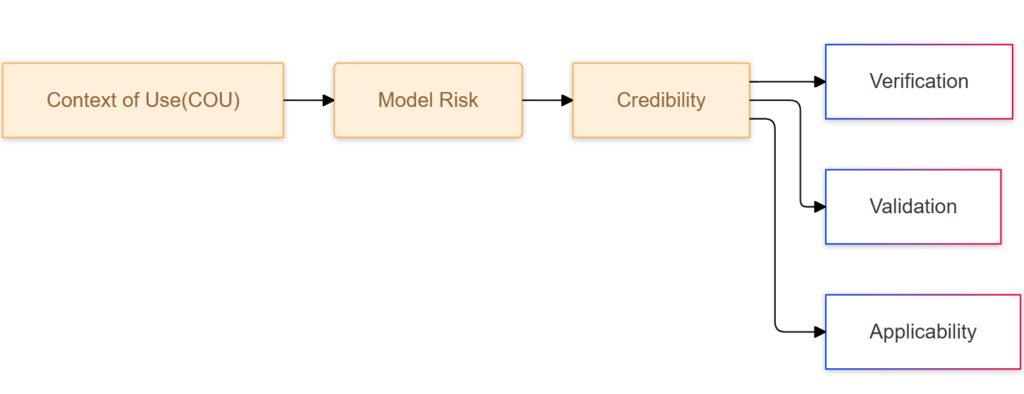
Context of Use
Context of Use (COU): The COU is a clear definition of the specific purpose and application of the computational model within the medical device development process. For example, if the model is used to develop a heart implant, the COU would specify that the model aims to simulate the implant’s behavior within the heart and predict potential issues that may arise after a certain number of heart beats.
Model Risk: A Critical Consideration
V&V40 places significant emphasis on assessing the potential risks associated with relying on computational models. Model risk encompasses two main components: model influence and decision consequence.
- Model Influence: This refers to the extent to which the model’s results contribute to the overall decision-making process in the medical device design. Other sources of scientific data are also considered when evaluating model influence.
- Decision Consequence: This component assesses the potential negative impact on patient safety if decisions made based on the model prove to be incorrect.
The interplay between these two components is crucial. A higher model influence combined with a significant decision consequence indicates a greater overall model risk.
Credibility
This sector is the hard work for engineers. It needs solid CM&S knowledge and experinece for verification and also practical experimental experience.
- Verification: This process focuses on ensuring the accuracy and correctness of the mathematical model and its calculations. It involves checking if the model is implemented correctly and if the mathematical equations and algorithms are solved accurately.
- Validation: This step involves comparing the model’s predictions against real-world experimental data. The goal is to confirm that the model can accurately represent the physical phenomenon it simulates. This process can be challenging, as it requires aligning the experimental design with the model’s parameters. Factors like sample selection, experimental procedures, and data collection methods must be carefully considered to ensure meaningful comparisons.
- Applicability: This element confirms that the chosen validation activities and the resulting evidence are directly relevant to the model’s intended use, as defined in the context of use (COU).
The Significance of Detailed Reporting
A comprehensive report is essential for effectively communicating the credibility of a computational model, especially when submitting evidence to regulatory bodies like the FDA. This report should transparently present the model’s development process, the verification and validation activities conducted, and the results obtained. Such transparency enables reviewers to understand and assess the model’s credibility and build trust in its reliability.
Embracing a Comprehensive Approach
V&V40 is not merely a template-based approach for documenting computational modeling results. It demands a thorough understanding of the principles of verification and validation. Moreover, it requires tailoring the documentation to each specific model and its intended use. The standard emphasizes a thoughtful and comprehensive evaluation rather than a simple data-filling exercise.
Challenges & Future Impact
Implementing V&V40 can be challenging, especially given the need for expertise in both computational modeling and experimental techniques. Effective collaboration and communication between simulation engineers and experimentalists are crucial for successful validation activities.
Despite the challenges, V&V40 has the potential to significantly shape the future of medical device development. By promoting the use of reliable and robust computational models, the standard can foster innovation. Furthermore, V&V40 can streamline regulatory approval processes by providing a clear framework for demonstrating model credibility. However, widespread adoption of V&V40, including comprehensive reporting practices, remains limited. Ongoing efforts to develop real-world case studies and resources are essential to promote broader understanding and application of the standard.
The ASME V&V40 standard provides a valuable framework for establishing the credibility of computational models used in medical device development. By understanding the standard’s principles and addressing the challenges associated with its implementation, medical device developers can harness the power of computational modeling to drive innovation while prioritizing patient safety and ensuring regulatory compliance.